Answer:
See explanation.
Step-by-step explanation:
Hello!
In this case, since the main molecular reaction that is taken place in the beaker is:
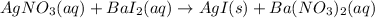
In such a way, we understand that one breaker contained silver nitrate and the other one barium iodide. Thus, the complete molecular equations turns out:
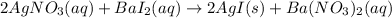
Now, for the complete ionic equation, we just ionize the aqueous species:
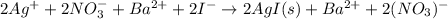
Finally, for the net ionic equation we cancel out barium and nitrate ions as the spectator one because they are both sides on the equation:
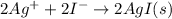
Best regards!