Answer: The correct answer is : 23.5 mol of O2 .
At STP ( Standard Temperature and potential ) 1 mole of gas has 22.4 L of volume .The follow can be expressed as :
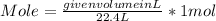
Given volume of O2 gas = 526 L
Plugging value in the formula :
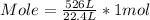
Mole of O2 gas at STP = 23.5 mol