Answer:
39.95 mL is the volume of the solute
13.77% is the volume percent (v/v).
Step-by-step explanation:
Density of solute = 1.15 g/mL
Mass of solute = 45.95 g
Volume of the solute = V
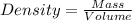
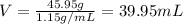
Volume of the solvent that water = 250 mL
Volume of the solution = 250 mL + 39.95 mL = 289.95 mL
Volume percent (v/v):
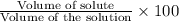
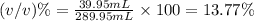
39.95 mL is the volume of the solute
13.77% is the volume percent (v/v).