Answer: D.
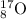
Explanation: Atomic number is the number of protons or the number of electrons.
Mass number is the sum of number of protons and number of neutrons.
Atomic number = number of electrons= number of protons = 8
Mass number = number of protons + number of neutrons= 8+9= 17
The representation of element is :
where Z= atomic number, A= mass number , X= chemical symbol of element
The representation of the element with atomic number 8 and mass number 17 is :
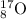 .
.