Answer:
1.72 grams is the mass of potassium hydroxide in the mixture.
Step-by-step explanation:
Total mass of the mixture = M = 5.50 g
Le the mass of KBr and KOH be x and y.
x + y = 5.50 g..[1]
Moles of KBr =
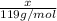
1 mol of KBr has 1 mol of potassium . Then
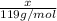 moles of KBr will have:
moles of KBr will have:
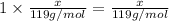
Mass of potassium in x amount of KBr :
=
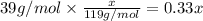 ...[2]
...[2]
Moles of KOH=
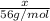
1 mol of KOH has 1 mol of potassium . Then
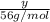 moles of KOH will have:
moles of KOH will have:

Mass of potassium in y amount of KOH :
=
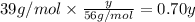 ...[3]
...[3]
Mass of potassium in the mixture = 2.45 g
0.33x+ 0.70y=2.45 g..[4] (from [2] and [3] )
On solving [1] and [4] we get:
x = 3.78 g, y = 1.72 g
1.72 grams is the mass of potassium hydroxide in the mixture.