Answer : The correct answer is : 61.13 g
Potassium chlorate decompose to give potassium chloride and oxygen . The balanced reaction is as follows :
2 KClO₃ → 2 KCl + 3 O₂
Given : Mass of KClO₃ = 100.0 g Mass of KCl = ?
Following are the steps to calculate mass of KCl :
Step 1: Convert mass of KClO₃ to its mole .
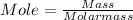
Molar mass of KClO₃ = 122.55
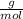
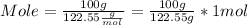
Mole = 0.82 mol
Step 2: Find mole ratio between moles of KClO₃ and KCl .
In balanced reaction the coefficients of KClO₃ is 2 and that of KCl is also 2. Hence the mole ratio of KClO₃ : KCl = 2 : 2 = 1 : 1 .
Hence mole of KCl =>
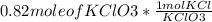 = 0.82 mol of KCl .
= 0.82 mol of KCl .
Step 3: Conversion of mole into grams .
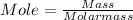
Molar mass of KCl = 74.55
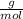
Plugging value in above formula :
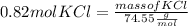
Multiplying both side by
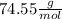 :
:
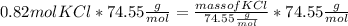
Mass of KCl = 61.13 g
Hence mass of KCl produced = 61.13 g