The mass of the acid : 75.64 g
Further explanation
Given
volume = 48.8 ml
density = 1.55 g/ml
Required
the mass
Solution
Further explanation
Density is a quantity derived from the mass and volume
Density is the ratio of mass per unit volume
Density formula:
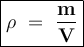
Input the value :
mass = ρ x V
mass = 1.55 g/ml x 48.8 ml
mass = 75.64 g