Answer: B: [C]
Explanation:
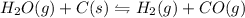
Equilibrium constant is defined as the ratio of the product of the concentration of products to the product of the concentration of reactants each raised to their stochiometric coefficient.
![K=\frac{[H_2][CO]}{[H_2O][C]]()
But the concentration of solids in their standard state is taken as 1.
Thus [C(s)] =1
![K=\frac{[H_2][CO]}{[H_2O]]()