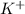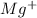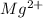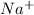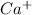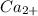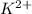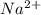The standard metal cations in soap are sodium
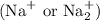 and potassium
and potassium
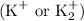 . These ions form soluble salts, contributing to the cleansing properties of soap.
. These ions form soluble salts, contributing to the cleansing properties of soap.
In the context of the active components of soaps, the relevant metal cations are typically sodium
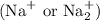 and potassium
and potassium
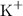 or
or
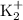 . These cations are commonly found in the form of sodium or potassium salts in soap molecules.
. These cations are commonly found in the form of sodium or potassium salts in soap molecules.
Other metal cations like magnesium
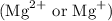 and calcium
and calcium
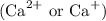 are generally not preferred for soap formulations because they can react with fatty acids in soap, forming insoluble compounds that reduce the effectiveness of the soap. Therefore, sodium and potassium salts are the standard metal cations in the active components of most soaps.
are generally not preferred for soap formulations because they can react with fatty acids in soap, forming insoluble compounds that reduce the effectiveness of the soap. Therefore, sodium and potassium salts are the standard metal cations in the active components of most soaps.
The complete question is:
Identify the standard metal cations in the active components of soaps. More than one cation can be selected if necessary.