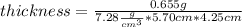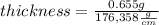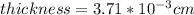Answer:
The thickness of the piece of foil is
Step-by-step explanation:
Please see the figure below that represents the aluminum foil. The volume of the tin foil will be:
V = length * width * thickness
Now, the density of tin is given by the expression:
ρ =
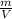
where m is the mass of the tin and V is the volume of it.
From the expression for the density we have:
V = m/ρ
And replacing the expression for the Volume of the tin, we have:
length*width*thickness = m/ρ
so
thickness = m/(ρ*length*width)
And replacing the given values we have: