Answer:

Step-by-step explanation:
The molar mass is the mass of 1 mole of particles in a substance. We can calculate using values on the Periodic Table.
First, identify the elements in the compound: SeOBr₂.
- Selenium (Se), Oxygen (O), and Bromine (Br)
Use the Periodic Table to find the molar masses.
- Se: 78.97 g/mol
- O: 15.999 g/mol
- Br: 79.90 g/mol
Notice the formula has a subscript of 2 after bromine. This means there are 2 moles of bromine in 1 mole of SeOBr₂.
To calculate the molar masses, multiply bromine's molar mass by 2, then add selenium and oxygen.
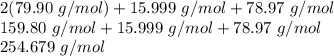
The molar mass of SeOBr₂ is 254.679 grams per mole.