Answer:
2:2:1
Step-by-step explanation:
The reaction expression is given as:
HXO → HX + O₂
We are to balanced this unbalanced reaction expression.
To solve this put, coefficient a, b and c before every specie:
aHXO → bHX + cO₂
Now use a mathematical approach to solve this;
Conserving H: a = b
X: a = b
O: a = 2c
let a = 1, b = 1 and c =
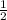
Multiply through by 2;
a = 2, b = 2 and c = 1
So;
2HXO → 2HX + O₂
Balanced ratio; 2:2:1