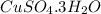Answer:
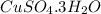
Step-by-step explanation:
Let us suppose there are x water molecules. Thus the formula of hydrated compound will be
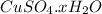
To calculate the mass percent of element in a given compound, we use the formula:
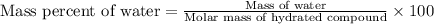
Given : Mass percent of water = 25.3 %
Mass of water =
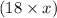 g
g
Molar mass of hydrated compound
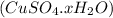 =
=
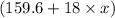 g
g
Putting in the values we get:
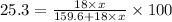
Solving for x we get:
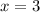
Thus the formula of hydrated compound will be