Answer:
Magnesium is being oxidized.
Titanium is being reduced.
Step-by-step explanation:
Hello,
The undergoing chemical reaction is:

Next, oxidation states are assigned:
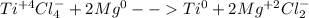
Half reactions are as follows:
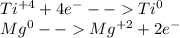
Finally, as the magnesium increases its oxidation state, it is being oxidized and as the titanium decreases its oxidation state it is being reduced.
Best regards.