Answer:
Energy released = 2832.79 kJ
Step-by-step explanation:
The standard enthalpy of the combustion of methanol,
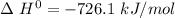
It means that :
1 mole of methanol on combustion releases 726.1 kJ of energy
Molar mass of methanol = 32.04 g/mol
Thus, mass of 1 mole of methanol is 32.04 g
So,
32.04 g of methanol on combustion releases 726.1 kJ of energy
1 g of methanol on combustion releases 726.1/32.04 kJ of energy
125 g of methanol on combustion releases (726.1/32.04)*125 kJ of energy
Energy released = 2832.79 kJ