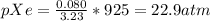Answer:
Partial pressure of Xe = 22.9 atm
Step-by-step explanation:
Given:
Total pressure of gas mixture = 925 atm
Mass of He = Ne = Xe = 10.5 g
To determine:
Partial pressure of Xe
Step-by-step explanation:
As per Dalton's Law in a mixture of gases the total pressure is the equal to the sum of partial pressures
In this case:
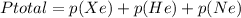 -------------(1)
-------------(1)
Partial pressure of each gas can be expressed as:
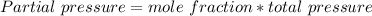
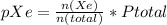 -----(2)
-----(2)
where n(Xe) = moles of Xe
n(Total) = total moles
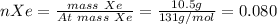
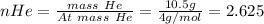
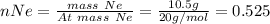
Therefore,
n(Total) = 0.080+2.625+0.525 = 3.23
Substituting for nXe, n(Total) and P(total) in equation (2)