Step-by-step explanation:
Atomic number of Xenon is 54 and its electronic configuration is
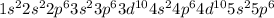 .
.
When an element gains an electron then it acquires a charge of -1. And, when two electrons are gained by an element then it acquires a charge of -2 and so on.
Tellurium is the element which has 52 electrons and electronic configuration of
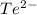 is as follows.
is as follows.
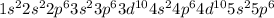
Thus, we can conclude that tellurium element forms an ion with an electronic configuration of
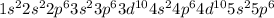 and a charge of -2.
and a charge of -2.