Answer : The solubility of
 is, 0.19 mole/L
is, 0.19 mole/L
Explanation :
The formula used for henry's law is:
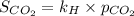
where,
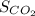 = solubility of
= solubility of
 = ?
= ?
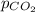 = partial pressure of
= partial pressure of
 = 5.6 atm
= 5.6 atm
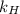 = Henry's law constant =
= Henry's law constant =
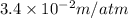
Now put all the given values in the above formula, we get:
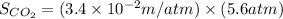
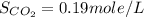
Therefore, the solubility of
 is, 0.19 mole/L
is, 0.19 mole/L