Answer: The correct answer is Option C.
Step-by-step explanation:
When a compressed gas is released from a tank, we are releasing the pressure or we are decreasing the pressure. With decrease in pressure, there will be increase in volume.
This relation is stated by Boyle's Law. This law states that pressure is inversely proportional to the volume of the gas at constant temperature and number of moles.
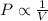
When pressure increases, volume will decrease and vice-versa.
Hence, in this case, the volume will expand and the compressed tank will burst open when the pressure is released quickly and will hit any object or person nearby it.
Therefore, the correct answer is option C.