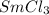Answer: The correct option is, (a)
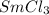
Step-by-step explanation:
(a)
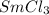
In this molecule, there are one number of samarium atom and three number of chlorine atoms. Hence, the total number of atoms present in
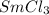 are, four.
are, four.
(b)
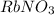
In this molecule, there are one number of rubidium atom, one number of nitrogen atom and three number of oxygen atoms. Hence, the total number of atoms present in
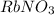 are, five.
are, five.
(c)
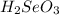
In this molecule, there are one number of selenium atom, two number of hydrogen atoms and three number of oxygen atoms. Hence, the total number of atoms present in
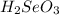 are, six.
are, six.
(d)
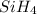
In this molecule, there are one number of silicon atom and four number of hydrogen atoms. Hence, the total number of atoms present in
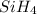 are, five.
are, five.
From the above we conclude that,
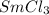 molecule contains the fewest number of atoms that is four than the other molecules.
molecule contains the fewest number of atoms that is four than the other molecules.
Hence, the correct option is, (a)