Let the formula of compound be
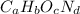 . The mass and molar mass of compound is 5.214 g and 129.1 g/mol respectively.
. The mass and molar mass of compound is 5.214 g and 129.1 g/mol respectively.
The combustion of compound gives 5.34 g of
 , 1.09 g of
, 1.09 g of
 and 1.70 g of
and 1.70 g of
 . First number of moles of carbon, hydrogen, oxygen and nitrogen to compare the molar ratio.
. First number of moles of carbon, hydrogen, oxygen and nitrogen to compare the molar ratio.
Calculation for number of moles:
For number of moles of C, first calculate number of mole of
 :
:
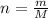
Here, m is mass and M is molar mass.
Molar mass of
 is 44.01 g/mol thus,
is 44.01 g/mol thus,
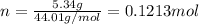
Since, 1 mol of
 have 1 mole of C thus, number of C will be 0.1213 mol.
have 1 mole of C thus, number of C will be 0.1213 mol.
Or,
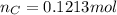
Convert this into mass as follows:
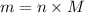
Molar mass of C is 12 g/mol thus,
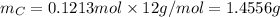
For number of moles of H, first calculate the number of moles of
 :
:
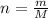
Molar mass of
 is 18 g/mol thus,
is 18 g/mol thus,
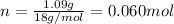
Since, 1 mol of
 have 2 mole of H thus, number of H will be 2×0.060 mol=0.12 mol.
have 2 mole of H thus, number of H will be 2×0.060 mol=0.12 mol.
Or,
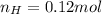
Convert this into mass as follows:
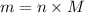
Molar mass of H is 1 g/mol thus,
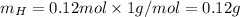
For number of moles of N, first calculate the number of moles of
 :
:
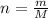
Molar mass of
 is 28 g/mol thus,
is 28 g/mol thus,
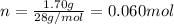
Since, 1 mol of
 have 2 mole of N thus, number of N will be 2×0.060 mol=0.12 mol.
have 2 mole of N thus, number of N will be 2×0.060 mol=0.12 mol.
Or,
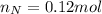
Convert this into mass as follows:
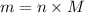
Molar mass of N is 14 g/mol thus,
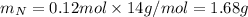
Since, mass of compound is 5.214 g thus, mass of oxygen will be:
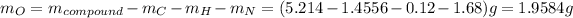
Molar mass of oxygen is 16 g/mol thus, number of moles of oxygen will be:
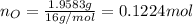
The ratio of number of moles of C, H,O and N will be:
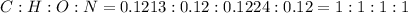
Thus, empirical formula of compound will be CHON.
According to above formula molar mass of compound will be 43 g/mol
Now, according to chemical formula, the molar mass is 129.1 g/mol taking the ratio:
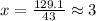
Thus, chemical formula will be
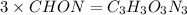
Therefore, chemical formula of compound is
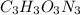 .
.