Answer: The density of metal is 9.28 g/mL
Step-by-step explanation:
Density of a substance is defined as the ratio of its mass and volume. To calculate the density, we use the equation:
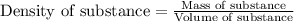 .......(1)
.......(1)
Mass of sample 1 = 39.87 g
Volume of sample 1 = 4.3 mL
Putting values in equation 1, we get:
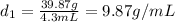
Density of sample 1,
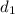 = 9.87 g/mL
= 9.87 g/mL
Mass of sample 2 = 30.84 g
Volume of sample 2 = 3.5 mL
Putting values in equation 1, we get:
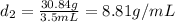
Density of sample 2,
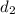 = 8.81 g/mL
= 8.81 g/mL
Mass of sample 3 = 38.46 g
Volume of sample 3 = 4.2 mL
Putting values in equation 1, we get:
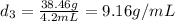
Density of sample 3,
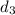 = 9.16 g/mL
= 9.16 g/mL
To calculate the density of metal, we take the average of the densities by using the equation:
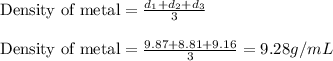
Hence, the density of metal is 9.28 g/mL