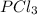Answer: The molecular formula of the compound is
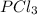
Step-by-step explanation:
Molecular mass of the compound is defined as the sum of the masses of all the individual atoms present in a compound.
We are given a molecular mass of 137.32 g/mol and we need to find the molecular formula for this molecular mass. For that we calculate the molecular mass of the given compounds individually and then match the given molecular mass.
Molar mass of phosphorous = 30.974 g/mol
Molar mass of chlorine = 35.453 g/mol
- Molecular mass of
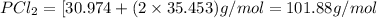
- Molecular mass of
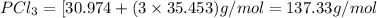
As, the molecular mass of
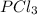 matches the given molecular mass.
matches the given molecular mass.
Hence, the molecular formula of the compound is