Step-by-step explanation:
Transition metals are defined as the metals that have incompletely filled d-orbital in their ground state or stable oxidation state.
General electronic configuration of transition metals is
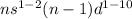 .
.
The transition elements which are present in period 4 are scandium, titanium, vanadium, chromium, manganese, iron, cobalt, nickel, copper and zinc.
Thus, we can conclude that the filling of d-orbital is represented by the transition metals in period 4.