Answer:
100.0 milliliters
Step-by-step explanation:
Using the dilution formula,
M₁V₁ = M₂V₂
Where M₁ and M₂ are the initial and final molarity, V₁ and V₂ are the initial and final volume.
V₁ = 250 ml
M₁ = 1.2 M
M₂ = 3M
Plugging in the numbers in the dilution formula we get,
1.2 M x 250 ml = 3M x V₂
V₂ =
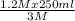 = 100 ml
= 100 ml
Therefore, 100 ml of a 3.0 M HCl solution are required to make 250.0 milliliters of 1.2 M HCl.