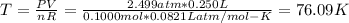Answer:
T = 76.09 K
Step-by-step explanation:
Given:
Moles of He, n = 0.1000
Volume, V = 250.0 ml = 0.250 L
Pressure, P = 253.25 kPa
To determine:
Temperature, T
Formula:
Based on the ideal gas law: PV = nRT -----(1)
R = 0.0821 L.atm/mol-K
P = 253.25 kPa
Now, 1 kPa = 0.00987 atm
Therefore, 253.25 kPa = 2.499 atm
Based on equation (1)