Answer:
Molarity:
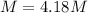
Molality:
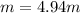
Percent by mass: %
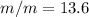 %
%
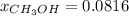
Mole percent:
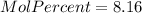 %
%
Step-by-step explanation:
Hello,
a.) Molarity: in this case, we first must compute the moles of methanol:
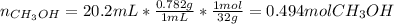
Then the volume of the solution:
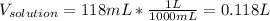
And the molarity:
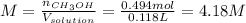
b.) Molality: in this case, we already computed the moles of methanol, so we just divide by the water's mass in kilograms:
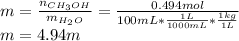
c.) Percent by mass: here, we compute the mass for both methanol and water as follows:
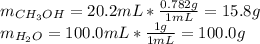
%
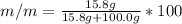 %
%
%
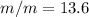 %
%
d.) Mole fraction: based on the densities and the measured volumes, one computes the moles of water because the methanol moles were already computed:
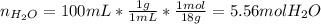
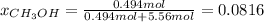
e.) Mole percent: here, we just multiply the mole fraction by 100% as follows:
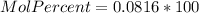 %
%
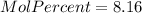 %
%
Best regards.