Density is equal to the ratio of mass to the volume.
Mathematical expression is given by:
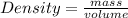 (1)
(1)
Mass of the rectangular object = 2.50 g
Volume =
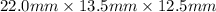
= 3712.5
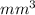
Now, convert
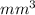 into mL
into mL
1
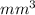 = 0.001 mL
= 0.001 mL
Thus, volume = 3.7125 mL
Put the values in formula (1)
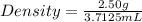
= 0.6734 g/mL
In three significant figures, density is equal to 0.673 g/mL
Hence, density is equal to 0.673 g/mL