Answer: Oxidation is defined as the reaction in which there is loss of electrons. It is accompanied by increase in oxidation number.
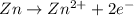
Zinc in solid state has oxidation number of zero and on losing electrons changes to
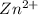 with oxidation number of +2.
with oxidation number of +2.
Reduction is defined as the reaction in which there is gain of electrons. It is accompanied by decrease in oxidation number.
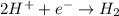
Hydrogen ions with +1 oxidation state gains electrons and converts to molecular hydrogen with oxidation state of zero.
In the given redox reaction, both oxidation and reduction takes place. They go hand in hand.
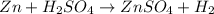
Zinc converts to
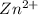 and gets oxidized.
and gets oxidized.
 gains electron and convert to
gains electron and convert to
 .
.