Answer : The number of moles of
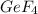 needed to produce 8 mole of
needed to produce 8 mole of
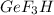 are, 6.48 moles.
are, 6.48 moles.
Explanation :
The given balanced chemical reaction is:
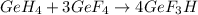
First we have to calculate the moles of
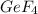
From the balanced chemical reaction we conclude that,
As, 4 moles of
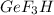 produced from 3 mole of
produced from 3 mole of
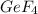
So, 8 moles of
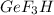 produced from
produced from
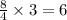 mole of
mole of
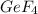
Now we have to calculate the moles of
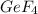 when the reaction yield is 92.6 %.
when the reaction yield is 92.6 %.
As, 92.6 % yield produced from 6 moles of
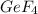
So, 100 % yield produced from
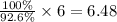 moles of
moles of
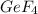
Therefore, the number of moles of
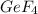 needed to produce 8 mole of
needed to produce 8 mole of
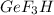 are, 6.48 moles.
are, 6.48 moles.