Answer: Bromine atom will have 7 valence electrons.
Step-by-step explanation:
Valence electrons are defined as the electrons which are present in the outermost shell of an atom known as valence shell.
Bromine is the 35th element of the periodic table having 35 number of electrons.
The electronic configuration of bromine is
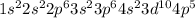
The valence shell for this element has n = 4. So, the number of electrons that are present in orbitals having n = 4 are 7.
Thus, bromine atom will have 7 valence electrons.