Answer: Cabon dioxide is always made of 8 parts of oxygen to 3 parts carbon by mass.
Step-by-step explanation:
Law of definite proportion states that the elements combining to form compounds always combine in a fixed ratio by their mass.
The chemical formula for carbon dioxide is
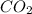
In 1 mole of carbon dioxide, 1 mole of carbon atom and 2 moles of oxygen atoms are present.
We know that:
Molar mass of carbon = 12 g/mol
Molar mass of oxygen = 16 g/mol
In carbon dioxide molecule:
Mass of carbon atom = 12 g
Mass of oxygen atom = (2 × 16) = 32 g
Mass ratio of oxygen and carbon in carbon dioxide molecule is:
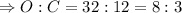
Hence, carbon dioxide is always made of 8 parts of oxygen to 3 parts carbon by mass.