Option 2: 12.0 L of
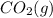 at STP.
at STP.
The standard pressure and temperature values are 1 atm and 273.15 K.
Using the ideal gas equation, number of moles of gas can be calculated which is as follows:
PV=nRT...... (1)
Here, P is pressure, V is volume, n is number of moles, R is gas constant and T is temperature.
Also, in 1 mole of any gas there are
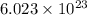 molecules of the gas. This is known as Avogadro's number and denoted by symbol
molecules of the gas. This is known as Avogadro's number and denoted by symbol
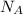
Thus,
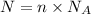
Equation (1) can be rewritten as follows:
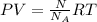
On rearranging,
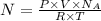
Here, all the other terms are constant except volume, thus, gas with volume equal to the volume of
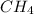 will have same number of molecules.
will have same number of molecules.
Volume of
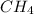 gas and
gas and
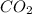 gas is same thus,
gas is same thus,
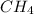 will have same total number of molecules as
will have same total number of molecules as
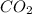 gas.
gas.