Answer:
It has a higher concentration of hydroxide ions than hydronium ions.
Step-by-step explanation:
A basic solution has pH value above 7, neutral solutions have pH equal to 7, and acid solutions pH value below 7.
The value of pH is
![-log[H3O^+]](https://img.qammunity.org/2019/formulas/chemistry/high-school/z7dafol0v2rnpcp33b8akzop1u8uikf9nm.png) , where
, where
![[H3O^+]](https://img.qammunity.org/2019/formulas/chemistry/high-school/fre71usdkhfl023j2s45tach0s7p3wz1dp.png) is the concentration of ion hydronium. As higher is its value, low will be pH. When water dissociates, besides hydronium, it forms hydroxide
is the concentration of ion hydronium. As higher is its value, low will be pH. When water dissociates, besides hydronium, it forms hydroxide
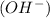 , so, for a basic solution, the concentration of hydroxide will be higher than the concentration of hydronium.
, so, for a basic solution, the concentration of hydroxide will be higher than the concentration of hydronium.
Some bases have a sour taste, some of them have an odor. So it's not possible to identify the solution by that.
As explained above, basic solutions release hydroxide ions in a reaction. Acids solutions release hydrogen ions or hydronium anions.