Answer:
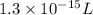 .
.
Explanation:
Volume of the gas is defined as the space occupied by a substance. It is expressed in units like
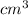 ,
,
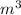 , L and ml.
, L and ml.
All these units of volume are inter convertible.
We are given:
Volume of the gas =
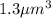
Converting this unit of volume into 'L' by using conversion factor:
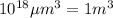
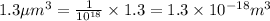
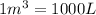
Thus

Thus the volume in Liters would be
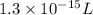 .
.