The latent heat of fusion of water at 0 °C is 79.7 calories/g.
This means 79.7 calories are required to melt 1 g of ice.
Thus, calories of heat required to melt 1.52 g of ice cube will be:
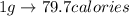
Thus,
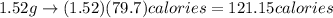
Thus, 121.15 calories are required to melt 1.52 g of ice cubes.