Avagadros number states that 1 mol of any substance is made of 6.022 x 10²³ units.
these units could be atoms, molecules or formula units.
therefore 1 mol of Calcium iodide is made of 6.022 x 10²³ formula units
so if 6.022 x 10²³ formula units make up 1 mol
then 1.20 x 10²⁵ formula units - make
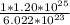 = 19.9 mol
= 19.9 mol
there are 19.9 mol of CaI₂ present