Answer : The correct option is, (3) (i) and (ii) only
Explanation :
According to the Brønsted-Lowry concept is that, a substance is a Brønsted-acid if it can donate a proton or hydrogen ion,
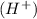 and a substance is a Brønsted-base if it can accept a proton or hydrogen ion,
and a substance is a Brønsted-base if it can accept a proton or hydrogen ion,
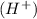 .
.
In the given options,
 and NaOH are Brønsted-base because they can accept a proton or hydrogen ion,
and NaOH are Brønsted-base because they can accept a proton or hydrogen ion,
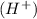 . While the other options are not a Brønsted-base because it can not accept a proton or hydrogen ion,
. While the other options are not a Brønsted-base because it can not accept a proton or hydrogen ion,
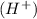 .
.
Hence, (i)
 and
and
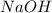 are Brønsted-base.
are Brønsted-base.