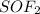
The name of the compound is thionyl fluoride.
Here, sulfur atom is the central atom with 6 valence electrons. It shares the two electrons to form double bond with oxygen atom and 1 electron each with the two fluorine atoms. The remaining two electrons will remain on the sulfur atom as lone pair.
The structure of the compound is attached here.