Answer:
Halogenation.
Step-by-step explanation:
Hello,
In this case, we must remember that group VIIA elements are known as halogens which include F, Cl, Br, I and At. Now, when an alkane reacts with the diatomic form of one of those halogen a haloganation chemical reaction occurs forming an alkyl halide and a hydrogen halide. For instance, for ethane, if it reacts with iodine, ethyl iodide and hydrogen iodide are yielded based on the given example:
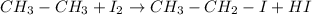
Best regards