Answer:
18.74 m/s is the root mean square speed of chlorine gas molecules under the same conditions.
Step-by-step explanation:
Average kinetic energy is defined as the average of the kinetic energies of all the particles present in a system. It is determined by the equation:
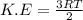
where,
K.E = Average kinetic energy
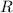 =Universal gas constant =8.314 J /mol K
=Universal gas constant =8.314 J /mol K
T = Temperature of the system
He has an average kinetic energy of 8750 J/mol
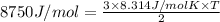
T =
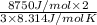
T = 701.63 K
The formula used for root mean square speed is:
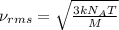
where,
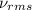 = root mean square speed
= root mean square speed
k = Boltzmann’s constant =
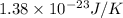
T = temperature =701.63 K
M = atomic mass = 0.071 kg/mole
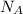 = Avogadro’s number =
= Avogadro’s number =
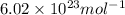
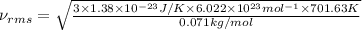
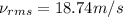
18.74 m/s is the root mean square speed of chlorine gas molecules under the same conditions.