Answer : The balanced equation will be,
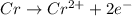
Explanation :
Oxidation : It is the process of losing of one or more electrons.
Reduction : It is the process of gaining of one or more electrons.
The balanced equation will be,
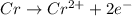
A neutral chromium atom is oxidized into chromium(II) ions by the loss of two electrons.