Answer:
The correct answer is 0.1236 M.
Step-by-step explanation:
What is the concentration of
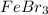 in a prepared solution by dissolving 10.0 g of
in a prepared solution by dissolving 10.0 g of
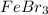 insufficient water to obtain 275 mL of solution?
insufficient water to obtain 275 mL of solution?
First, we must look for the molar mass of iron bromide (III).
Mm = 295.56 g/mol
Then, the amount of
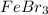 moles in 10 g is equal to:
moles in 10 g is equal to:
n=
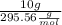
n= 0.034 mol
Now to know the molar concentration we have to divide the number of moles of the compound by the volume of the solution in liters:
Volume=
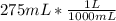
M=
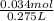
M=0.1236
Have a nice day!