Answer:
Freezing point of seawater is lower as compared to pure water
Step-by-step explanation:
Seawater contains salts like
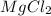 , NaCl etc.
, NaCl etc.
According to colligative properties of molecules, presence of solute in a pure solvent lowers freezing point of pure solvent obeying the following equation-
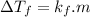
where
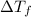 is lowering of freezing point,
is lowering of freezing point,
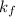 if cryogenoscopic constant of solvent and m is molality of solution.
if cryogenoscopic constant of solvent and m is molality of solution.
In seawater, water is the solvent and
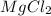 , NaCl etc are solutes.
, NaCl etc are solutes.
Hece freezing point of seawater is lower than pure water