Answer:
The solution of salt water will have a higher conductivity than the solution of sugar water.
Step-by-step explanation:
Sugar is an organic compound, made of covalent bonds between atoms.
Salt is an inorganic compound, made of ionic bond between ions.
Water is an inorganic solvent. So it can break the ionic bonds in inorganic compounds.
When salt is dissolved in water, it dissociates itself into its constituent ions. The sodium anion
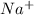 and chlorine cation
and chlorine cation
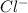 are made free and dispersed randomly in the water. Now the solution has free ions so it can conduct electricity.
are made free and dispersed randomly in the water. Now the solution has free ions so it can conduct electricity.
When the sugar cube dissolves, the individual sugar molecules are made free to move randomly in water but they do not break down into their constituent atoms. The sugar molecules do not have a charge on them (they are not ions). So, even though they are moving freely they cannot conduct electricity. Since the pure water does not conduct electricity, adding sugar will not alter its conductivity.