Answer: 4. equal to the rate at which ozone is formed.
Step-by-step explanation:
Characteristics of chemical equilibrium:
1. Chemical equilibrium are attained is closed system.
2. The macroscopic remains constant like: volume, pressure, energy etc.
3. The equilibrium is dynamic in nature and the reactions are continuous in nature. Rate of forward reaction is equal to the rate of backward reaction.
4. The concentration of the reactants and products remain constant.They are not always equal.
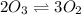
rate of decomposition of ozone = rate of formation of ozone