Answer:
18.43 Torr is the pressure of this substance after 70seconds.
Step-by-step explanation:
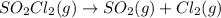
Rate of the reaction ,k=
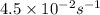
Integrated rate equation for first order kinetics in gas phase is given as:
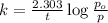
p= pressure of the gas at given time t.
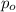 = Initial pressure of the gas
= Initial pressure of the gas
When, t = 70 sec
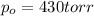
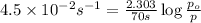
p = 18.43 Torr
18.43 Torr is the pressure of this substance after 70 seconds.