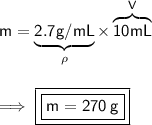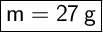

- Step-by-step explanation:
To find the mass of the aluminium, knowing its density and the volume it occupies, we can use the following formula:
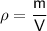
Where:
- ρ is the density.
- m is the mass.
- V is the volume.

Rearrange the formula to express m in terms of ρ and V:
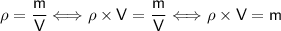

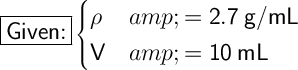

Substitute these values into our formula: