Answer:
If there are 10.0 g of sucrose and 8.0 g of oxygen, 0.029 moles of sucrose are available for this reaction
Step-by-step explanation:
The complete question is:
"The equation represents the combustion of sucrose.
C₁₂H₂₂O₁₁ + 12 O₂ ---> 12 CO₂ + 11 H₂O
If there are 10.0 g of sucrose and 8.0 g of oxygen, how many moles of sucrose are available for this reaction?
A.) 0.029 mol
B.) 0.250 mol
C.) 0.351 mol
D.) 3.00 mol"
To know the amount of moles ordered, you must know the molar mass of sucrose. For that you know the atomic mass of the elements that form it, obtained from the periodic table:
- C: 12 g/mol
- H: 1 g/mol
- O: 16 g/mol
Taking into account the abundance of each element, the molar mass of sucrose is:
C₁₂H₂₂O₁₁=12* 12 g/mol +22* 1 g/mol + 11* 16 g/mol= 342 g/mol
To know the mole amount of sucrose available for the reaction, a rule of three applies as follows: if 342 g of sucrose are present in 1 mole of the compound, 10 g of sucrose how many moles do they represent?
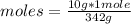
moles=0.029
If there are 10.0 g of sucrose and 8.0 g of oxygen, 0.029 moles of sucrose are available for this reaction