Answer : The correct option is, Chlorine
Explanation :
The given elements are, Se, B, Ca, Cl
Atomic number = 34
Group number = 16
General Electronic configuration of the group number 16 elements is,
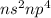 .
.
The general configuration shows the number of valence electrons are present in the element.
The number of valence electrons present = 2 + 4 = 6
Atomic number = 5
Group number = 13
General Electronic configuration of the group number 13 elements is,
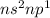 .
.
The number of valence electrons present = 2 + 1 = 3
Atomic number = 20
Group number = 2
General Electronic configuration of the group number 2 elements is,
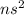 .
.
The number of valence electrons present = 2
Atomic number = 17
Group number = 17
General Electronic configuration of the group number 17 elements is,
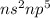 .
.
The number of valence electrons present = 2 + 5 = 7
From this we conclude that the chlorine is the element which has the greatest number of valence electrons available for bonding.
Hence, the correct option is, Chlorine.